Aerosol safety:
Introduction:
Aerosol cans are widely used for delivering personal care products,paints,lubricants,insect repellent, gardening and automotive goods to consumers.They are stored in general purpose warehouses,store rooms, retail outlets, and in workplaces where they are usually stored in cupboards and open shelves.
When exposed to fire, aerosol cans will either violently rupture or produce burning jets of flame. Both conditions expose adjacent aerosols and can result in more container failures. The only effective method of controlling this type of fire is the delivery of large quantities of water by sprinklers. If adequate protection is not provided, an aerosol fire will grow quickly and produce high temperatures, thick smoke and rocketing containers that can trail burning liquid to areas well away from the fire’s origin.
Aerosol cans consist of steel / aluminum containers fitted with a plastic valve designed to discharge their contents as a fine mist, spray or stream. They usually contain liquid or powder product and a liquefied petroleum gas (LPG) propellant. The propellant creates the pressure necessary to eject the liquid or powder product in the desired form and is flammable. In many cases the liquid content is also flammable. Aerosol containers are designed to contain the pressure generated by the LPG at
temperatures below 54°C.
When exposed to flame, aerosol containers experience temperatures and pressures significantly
higher than they were designed to resist, causing them to rupture violently into large fire balls if the
pressure is not vented. This occurs when the gas and liquid inside expands when heated increasing
the pressure inside the container until the container cannot hold it any more and fails (ruptures). The
contents of the can may be a flammable liquid that is vaporized by the heat and loss of containment.
This gas or vapor may ignite causing an explosion or flash fire. This is known as a BLEVE, a Boiling
Liquid/Expanding Vapor Explosion.
Some aerosol containers have a valve designed to melt in a fire and vent the pressure; these types of containers produce flaming jest that can last for several minutes. This may not cause the can wall to rupture, but if the product or the propellant are flammable, it can also still result in a fireball.
(Examples of an exploding aerosol can under BLEVE conditions)
What is an aerosol?
An aerosol can be defined as a system of solid or liquid particles suspended in air or other gaseous environment. Aerosols vary in size and composition, they can be naturally or man made generated, and thus there are a wide range of them, from flame synthesized nano particles and nano materials (good aerosols), with fundamentally new properties and functions because of their small size (<100 nm) to airborne particulate matter resulted from the industrial production of nano materials, and viruses that have a negative effect in visibility and human health (bad aerosols).
Sources of aerosols:
Aerosols can be naturally or man made generated. The latter can also be produced inadvertently as a portion of combustion emissions, but they are also produced intentionally for commercial uses. Manufactured aerosols (nano particles and nano materials) are playing an increasing role in water, soil, and air treatments; efficient energy production and storage;
About three-quarters of all aerosols found in Earth's atmosphere come from natural sources. The most important of these natural components are sea salt, soil and rock debris, products of volcanic emissions, smoke from forest fires, and solid and liquid particles formed by chemical reactions in the atmosphere.
Forest fire:

Volcano:
Image of eruption of Mt. St. Helens in 1980. Volcano eruption is an example of an aerosol produced by natural causes. While smoke from volcano eruption can have severe effects on the respiratory system and cause damage to private and public property in the surroundings of the volcano, it is considered as a part of the ecosystem and cannot be prevented. However the secondary effects could be prevented by techniques based on aerosol science, for example using nano structured materials as absorbents to selectively capture undesired metals and other pollutants the atmosphere.
Image of aerosol produced by welding, and industrial and vehicle exhausts. These are examples of man made bad aerosols derived from combustion emissions and other processes. When large concentrations of nano particles may be present in occupational environments; and more studies need to be carried on in order to understand the effects of nano scale particles produced unintentionally in those environments.
The resulting information can be used to create new methodologies for reducing concentration of undesired nano scale particles in occupational environments. The phenomena occurring in aerosol route synthesis of nano particles are comparable to those in high temperature environment processes such as welding, and combustion exhausts; in both cases the particles are formed by nucleation. Thus aerosol techniques might be good approach to prevent the negative effect of those emissions. Furthermore, recent studies show that silica nano particles produced by an aerosol route can be used as sorbents to capture heavy metal resulting from combustion environments.
Car

Welding

Industry

Classification:
Aerosols are commonly classified into various subgroups based on the nature and size of the particles of which they are composed and,to some extent,the manner in which the aerosol is formed. Although relatively strict scientific definitions are available for each subgroup, these distinctions may become blurred in actual practical applications. The most important of these subgroups are fumes, dusts, mists, and sprays.
Fumes:
Fumes consist of solid particles—ranging in size from 0.001 to 1 micron—suspended in a gas. Probably the most familiar form of a fume is smoke. Smoke is formed from the incomplete combustion of fuels such as coal, oil, or natural gas. The particles that make up smoke are smaller than 10 microns in size.
Dusts:
Dusts also contain solid particles suspended in a gas, usually air, but the particles are larger in size than those in a fume. They range from about 1 to about 100 microns in size, although they may be even larger. Dust is formed by the release of materials such as soil and sand, fertilizers, coal dust, cement dust, pollen, and fly ash into the atmosphere. Because of their larger particle size, dusts tend to be more unstable and settle out more rapidly than do fumes, which do not settle out at all.
Mists:
Mists are liquid particles-less than about 10 microns in size-dispersed in a gas. The most common type of mist is that formed by tiny water droplets suspended in the air, as on a cool summer morning. If the concentration of liquid particles becomes high enough to affect visibility, it is then called a fog. A particular form of fog that has become significant in the last half century is smog. Smog forms when natural moisture in the air interacts with human-produced components, such as smoke and other combustion products, to form chemically active materials.
Sprays:
Sprays form when relatively large (10+ microns) droplets of a liquid are suspended in a gas. Sprays can be formed naturally, as along an ocean beach, but are also produced as the result of some human inventions such as aerosol can dispensers of paints, deodorants, and other household products.
Physical properties:
The physical and chemical properties of an aerosol depend to a large extent on the size of the particles that make it up. When those particles are very large, they tend to have the same properties as a macroscopic (large size) sample of the same material. The smaller the particles are, however, the more likely they are to take on new characteristics different from those of the same material in bulk.
Aerosols tend to coagulate, or to collide and combine with each other to form larger bodies. A cloud, for example, consists of tiny droplets of water and tiny ice crystals. These particles move about randomly within the cloud, colliding with each other from time to time. As a result of a collision, two water particles may adhere (stick) to each other and form a larger, heavier particle. This process results in the formation of droplets of water or crystals of ice heavy enough to fall to Earth as rain, snow, or some other form of precipitation.
Regulations and Standards:
Aerosol cans are classified as Dangerous Goods under Class 2, with a listed international reference number of UN1950. They are mainly identified with a red flammable gas diamond or in some cases, a flammable liquid diamond on the can.
Note: New Global Harmonised Labelling has been introduced and will replace the class diamondlabels over the next few years.
Under all state Dangerous Goods Storage and Handling Regulations it makes reference to the
placarding requirement for the various classes and divisions of dangerous goods. In respect of
Aerosols the placarding exemption limit is 5000L. Therefore it is not a requirement to placard a
premises, if they have 4999L or 16600 cans (of 300ml capacity) or less. That is still a lot of cans that
does not have to be identified for. However they still have to be safely stored.
There is very little detail relating to the storage of aerosols any of the respective State Regulations. However some states do provide information publications that do give some guidance for the storage of minor quantities of aerosols.
Impacts on Health:
Aerosol particles can contribute to a variety of human health problems. Particulate matter affects the health of more people than any other pollutant worldwide. According to estimates from the World Health Organization (WHO), particle pollution contributes to approximately 7 million premature deaths each year, making it one of the leading cause of worldwide mortality.
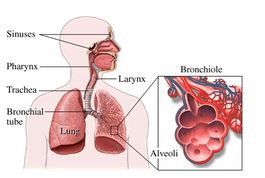
The health effects of the particles are directly related to their size. Particles greater than 100 μm are generally too large to be inhaled. Particles between 10-100 μm can be inhaled, but are usually stopped by our body’s “filters”— the mucus membranes in our respiratory system. The term inhabitable particles typically refers to particles less than 10 μm in size that can make it past the body’s defenses and deep into the lungs. Inhalable coarse particles are particles between 2.5 and 10 μm in diameter (PM10) and are typically found near highways and factories. As the figure to the right shows, these particles are usually deposited in the nose, pharynx, and larynx.
Particulate matter penetration into the human respiratory system. Based on their size, particles can penetrate into different parts of the human respiratory system. The “deposition fraction” is the fraction of particles of a certain size that is deposited, or gets stuck in, a certain part of the respiratory system. Most large particles are deposited in the nose, pharynx, or larynx, and therefore don’t make it deep into our lungs. Smaller particles, however, are more likely to penetrate more deeply into our lungs, into the trachea, bronchioles, or alveoli.
Suitability of wastes for on-site storage and treatment:
Canisters, such as those classified under the waste codes given above, may be accepted at waste facilities for storage for treatment or transfer. However, the specific waste canisters a site can accept will depend upon the potential hazards of the waste, the storage and treatment measures provided at the facility and, where applicable, will be defined by the conditions of the environmental permit held by the facility.
The propellant contained in a canister can typically range from 5% to 95% of the total content, and is used to provide the spray energy. It can also act as an essential component of the product, such as the foaming agent in shaving foam, or as the solvent in spray paint. Canister propellants include liquefied gases, such as hydrocarbons (typically propane or butane), di-methyl ether (primarily in cosmetic products), and compressed gases, such as carbon dioxide and nitrogen. Due to their ozone-depleting potential, CFCs have not been used in aerosol products manufactured in the UK since 1989. The use of hydrofluorocarbons (HFCs) as a propellant has also been limited due to their high global warming potential (HFCs are 150-1500 times more potent than carbon dioxide (CO2)). HFCs are only used in products when there are no viable alternatives (e.g. when they must be used to ensure the safety of a non-flammable product).
The contents of canisters can pose a wide range of dangers and may be marked with a variety of warnings, such as:
- Extremely flammable.
- Pressurised container: protect from sunlight and do not expose to temperatures exceeding 50°C.
- Do not spray on a naked flame or any other incandescent material.
- Keep away from sources of ignition- No smoking.
- Do not burn or puncture even after use.
- Use only in well ventilated areas.
- Solvent abuse can kill instantly (the “SACKI” warning)
Canisters containing water-based products may not burn easily in a fire, whereas those containing highly flammable products, such as an engine ignition fluid, will burn readily and feed the fire. Regardless of their content, however, all canisters are under pressure and therefore pose a potential danger, and must be handled and stored on-site in a safe manner.
Source-segregated or bulk loads of waste canisters should be directed to specialist canister treatment facilities, rather than metal recycling facilities or other material recovery facilities. At some canister treatment facilities, for example those that employ an automated plant provided with an appropriate system for gas collection or abatement, partially full or full canisters may be accepted for treatment, whereas at others it may only be appropriate to treat fully discharged (empty) canisters. If full or partially full canisters are received at a facility that is not appropriate for their treatment they should be segregated, stored safely and transferred to an appropriate facility for treatment.
The acceptance, storage and treatment of unsuitable waste canisters, or the storage and handling of canisters in inappropriate containers, can pose significant risks to health, safety and the wider environment. To a lesser extent, the treatment of inappropriate wastes can damage plant infrastructure and also increase the downtime of the treatment equipment, for example due to the time taken to clean out a foam product released inside the equipment. Therefore, it is important that waste acceptance criteria and plant design capability are based on clear specifications, taking into account variations in canister size and design and the quantity and nature of the canister content (i.e. the residual aerosol product and propellant), and that waste producers and site operatives are informed of the types of waste canisters that the facility can accept for storage for treatment or transfer. It is also essential that the Operator confirms (i.e. by visual inspection) that the waste received on site is consistent with what is expected and what can be accepted. These requirements should be achieved through the development and implementation of thorough waste pre-acceptance and acceptance procedures at the facility.
Hidden Dangers of Aerosol Cans:
We use them around the house for everything from touching up patio furniture, to dusting furniture, to making the air (or people) smell better. You can find them on nearly every jobsite, in most work vehicles, and in offices. They’re small and easy to ignore. But when they explode or depressurize incorrectly, they can be deadly.
Since its introduction as a device for dispensing insecticides in the jungles of the Second World War, what was known then as a “bug bomb” and today as aerosol cans have become widely used devices for a broad variety of applications. They’re portable and disposable, making them very convenient. Most workers don’t think twice about using an aerosol can and tossing it aside when the task is complete or the can is empty.
Aerosol cans are normally manufactured from thin sheets of steel. The products they hold are highly pressurized with a number of types of hydrocarbon propellants, from carbon dioxide or butane or propane. In recent years, some scientists and environmental activists have linked chlorofluorocarbon (CFC) propellants to decreases in the planet’s ozone layer. Most manufacturers have shifted to propellants that are thought to be less damaging to the atmosphere.
Interestingly, most products sold in aerosol cans carry much higher costs by weight or volume than their non-aerosol counterparts. The propellant in an aerosol can may account for as much as 15 percent of the weight, and when you compare the cost of the remaining material (such as paint) with other delivery methods (such as paint sold in regular cans), you’ll see that using aerosols nearly always involves a significantly higher cost.
Safe work practices:
As with most hazards, the first steps in reducing the dangers associated with aerosol cans is to determine whether they are really needed on the jobsite. If the task can be accomplished without the use of aerosol cars, workers will not have to contend with the hazards. Other forms of the material may be available. Or, refillable spray bottles or air-powered equipment may be available.
If workers do use aerosol cans, they should be familiar with the Material Safety Data Sheets (MSDS) for the material and use the cans according to directions. Personal protective equipment or additional ventilation may be required.
Aerosol cans should always be stored in dry areas where they will not be exposed to excessive temperatures. As the temperature rises, pressure in the can will increase, and ambient temperatures about 120 degrees Fahrenheit may lead to explosions. Because car and truck interiors can become very hot in sunlight (even during the winter months), vehicles are generally not a safe location for even temporary storage.
Disposal issues:
As noted earlier, leftover materials in partially filled cans may qualify as hazardous waste. If a can is found to be inoperative or malfunctioning, returning it to the supplier will prevent the user from having to treat it as hazardous waste.
Cans that are completely empty of both propellant and product are not considered to be hazardous waste, and may be recyclable. Companies that use a significant number of aerosol cans may wish to consider aerosol-puncturing equipment, which allows the contents of cans to be safely removed and prepared for disposal.
Aerosol cans should never be placed in fires or heated locations, because they may explode, and the propellant may be flammable. Cans that are still pressurized may also burst if place in a garbage compactor.
If cans that contain hazardous wastes are to be disposed, they should be placed in a special closed container displaying markings indicating that the waste is hazardous. The labeling should also indicate the specific types of waste and the date when the container began to be used. Keep records of when and how the waste was disposed or recycled.
Waste storage & handling:
Most aerosols contain materials which are a low hazard to the environment, indeed most are intended to release their contents just about anywhere. The risks if any, come mainly from fire which spreads to involve other materials. Aerosol cans are thin and will rust through quickly in the open air. If a fire starts in a stack of boxes it can be expected to spread quickly, with canisters ejected as they overheat. Some distribution sites place them in cages to prevent ‘missiles’. Indoor storage should be employed, to restrict the rate of rusting, and missile risk. An assessment should be undertaken to ensure that land around the store contains nothing that would be expected to be ignited by the contents of an ejected burning can, and to prevent fire spread by radiant heat on an adjacent stack if containment is compromised.
Due to the potential hazards posed by canisters (i.e. pressurised vessels often containing flammable gas propellant and liquid product), it is important that all appropriate measures are implemented at a facility to ensure that they are stored safely on site and to minimise risks (e.g. of explosion and propagation of fire) to an acceptable level.
Figure-1 Figure-2
Storage containers:
- Storage of canisters must take place under cover in secure well ventilated containers or within caged storage areas, in a well vented location which is not subject to extreme temperatures or direct sunlight.Good and bad examples of containers used to store canisters on-site are shown in Figure 2.
- Canisters received and held in insecure or flammable containers/packaging (e.g. in cardboard boxes, shrink-wrapped on pallets) must be stored in cages or transferred to secure ventilated containers to prevent the risk of them spreading fires by ‘missiling’ or ‘ejection’.
- Canisters held in containers that are not able to collect and hold liquids released from the canisters should be provided with suitable containment measures (e.g. drip trays) or transferred to secure containers that are able to retain free liquid.
Figure-3
- During storage, lids on containers holding canisters should remain securely closed at all times when not being filled, emptied or internally inspected and the doors/hatches of cages should remain closed and locked when not being used.
- Containers used to store canisters should not be over-filled. Over filling can result in canisters being actuated and discharging their contents, either under the weight of the canisters above them, when the container lid is closed or when containers are stacked.
- Cages used for storage should be robust, fire-resistant and of an appropriate mesh size (based upon the size of the canisters to be stored) to constrain the canisters and prevent any ejection. Where the cage is not constructed with a mesh roof, the mesh wall panels should extend into the roof space of the storage area to ensure that the structure is completely enclosed.
- Flammable liquids should not be collected or held in plastic drums or non-conductive plastic IBCs. Containers used to collect and hold flammable liquids from the treatment process should preferably be constructed from steel, or at least anti-static plastic, and designed so that they can be sealed for handling/storage purposes. Anti-static plastic containers and IBCs should only be used to collect and hold flammable liquids if they are held within a self-contained bund and segregated from other dangerous substances (e.g. other flammable materials, oxidisers or corrosive materials).
Aerosol can management:
Waste aerosol cans for scrap metal recycling, as non-hazardous solid waste, without first puncturing and draining them?
Yes, if the waste aerosol cans meet the following:
- The cans did not hold chemical formulations with sole active ingredients identified in the F027 or P-list hazardous waste listings, and
- The aerosol products have been used for their intended purposes so that when holding the cans upright and pressing down on their nozzles, not enough product comes out for them to be useful anymore, and
- a) No more than 3% of the original net content weight remains in the cans, or b) No more than one inch of liquid remains in the bottoms of the cans. A practical test is to shake the can up and down. If the can feels like it has an inch or less of liquid in the bottom when you shake it, it should meet this criterion.
Most aerosol cans have a round (concave) bottom. The one-inch criterion assumes a flat bottom.
Do not spray aerosol cans for the sole purpose of making them non-hazardous solid waste. Doing so:
1) may meet the definition of hazardous waste treatment and may require a hazardous waste treatment facility license, and
2) may generate wastes, which would require the generator to determine if they are hazardous waste and manage them accordingly.
These criteria should only be applied to waste aerosol cans while they are generated, transported or recycled
Waste treatment processes:
Compactors:
The process is sealed and operated under vacuum, preventing emissions to air.Canisters areinitially tipped into the hopper and an inert gas is used to prevent the formation of an explosive atmosphere and provide fire suppression. Hydraulic rams squash the canisters into briquettes forcing the release the liquid contents and gases. The gases are collected, recompressed and stored in tanks. The liquids are collected and piped to an IBC.
Shredders:
A conveyor feeds the canisters into the top of the shredder. Once shredded, the metal falls out of the bottom of the shredder and is collected. Liquids released from the canisters pass through the shredder and are collected in an IBC. The shredder may be fitted with nitrogen injection, explosion relief doors and a fire detection / CO2 suppression system. However, unlike the sealed vacuum processes, the shredder system is unlikely to be able to collect the gases released from the treated canisters.
AEROSOL SPRAY CANS:
DO's
- DO use up the contents of an entire spray can before starting another. Make sure that the can is completely empty before discarding it.
- DO return spray cans that malfunction (for example, the tip breaks off).
- DO use refillable mechanical spray cans when possible.
- DO establish a distribution control system to limit aerosol cleaner use.
- DO consider phasing out the use of spray cans in your shop.
DON'Ts
- DON'T spray in/or around other solvents. Hazardous contamination may result.
- DON'T discard partially empty spray cans in the trash dumpster.
- DON'T inhale the contents of the aerosol can.
- DON'T refill the aerosol can;
- DON'T puncture or burn the used aerosol can.
- DON'T smoke or use the aerosol can in a confined area where electrical appliances are in operation;
Click the below link to download the aerosol safety presentation

Power point presentation - Aerosol from Hazards
Power point presentation - Aerosol can explosion